Dipeptides
L-α-dipeptides (dipeptides) have not been studied nearly as much has have proteins and amino acids. The primary research has been done on L-aspartyl-L-phenylalanine methylester (aspartame) and Ala-Gln (Lalanyl-L-glutamine) because they are used in popular commercial products. In addition to this fact, another reason many dipeptides have not been studied thoroughly is because dipeptide production lacks effective production processes, even though several chemical and chemoenzymatic methods have been reported.
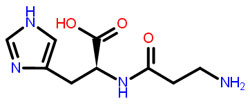
Carnosine - example of dipeptide
Until recently, new methods have been developed for dipeptide synthesis for which dipeptides are produced via fermentative processes. Certain dipeptides have distinctive physiological capabilities, permitting them to possibly hasten dipeptide applications in various fields of scientific research. L-α-dipeptides are comprised of the most uncomplicated peptide bond of two amino acids, yet they are not readily available primarily due to the exiguity of cost-effective processes of manufacturing. Dipeptides, however, have very interesting functions, and the scientific information surrounding them is increasing. This leaves many researchers with the charge of developing more efficient and cost-effective processes of dipeptide production. When this field is more fully studied, it is anticipated that we can learn much more about how valuable peptides really are.
Dipeptides have two basic functions, which are:
1. A derivative of amino acids
2. The dipeptide itself
As a derivative of amino acids, dipeptides, along with their amino acids contain different physiochemical properties, but they usually share the same physiological effects. This is because dipeptides are degraded into the separate amino acids in living organisms, which have varying physicochemical properties. For instance, L-glutamine (Gln) is heat-labile, while the Ala-Gin (L-alanyl-L-glutamine) is heat tolerant.
Chemical synthesis of dipeptides occurs as follows:
1. All functional dipeptide groups are protected (other than those that are involved in creating the peptide bond of amino acids).
2. The protected amino acid of the free carboxyl group is activated.
3. The activated amino acid reacts with the other protected amino acid.
4. The protecting groups contained within the dipeptide become removed.
Advantages and Disadvantages of Dipeptide Synthesis
One of the key advantages of chemical dipeptide synthesis is the ability to synthesize a wide variety of dipeptides through careful selection of appropriate protecting groups and activating reagents. Additionally, the process typically yields high product yields and is relatively straightforward to execute. However, the synthesis must be carried out step by step. The disadvantages of chemical dipeptide synthesis include the relatively high cost due to the multiple reaction steps required to create a dipeptide and form the peptide bond. Dipeptide synthesis methods can be classified into three different approaches:
• Chemical synthesis
• Enzymatic synthesis
• Chemoenzymatic synthesis - in this approach, the process involves using an enzyme in combination with at least one protected amino acid substrate.
According to research conducted by Gulewitsch and Amiradzibi (1990) and Hines and Suftin (1956), carnosine (β-alanyl-His) and the related dipeptide anserine (β-alanyl-N-methyl-His) are found in various mammalian, avian, and fish tissues. These dipeptides are thought to perform a host of biological functions, including:
• Antioxidant activity (Guiotto et al. 2005)
• Cellular pH regulation (Begum et al. 2005)
A Closer Look at Histidine Dipeptides
Dipeptides that contain histidine are a family of soluble peptides. Consisting of a histidine (or a histidine-like amino acid), these dipeptides also contain an imidazole ring and an atypical amino acid (e.g. β-alanine or) at the N-terminus of the peptide. The imidazole ring appears to be crucial for several of the biological roles that are assumed to exist in this family of dipeptides. They are known to be tissue- and species-specific.
Taking a closer look at carnosine, which is the primary dipeptide in this family, it received its name from the Russian scientist, Gulewitsch, who is credited for its discovery in meat. Meat in Latin is carnos. At any rate, these histidine-containing dipeptides are all members of this group of dipeptides, and carnosine was the first to be discovered. Relative to carnosine, though with different structures, are:
• Methylation on either p (anserine)
• s (balenine) nitrogen on the imidazole ring of histidine
• Substitution of c-aminobutyric acid for b-alanine (homocarnosine)
• Acetylation of the terminal amino group of b-alanine (N-acetylcarnosine)
Further, it is anticipated by the scientific community that histidine-containing dipeptides may fine-tune biological functions concordant to the biological roles and needs that do vary in tissue-specific manners. These modifications are species and tissue-specific. Depending upon the metabolic needs of glycolytic muscle fibers, histidine dipeptides are highly present in fast twitches of this muscle.
Carnosine has a metal-binding relationship and is used in the carnosine-zinc complex, which has been found to have membrane-stabilizing capabilities, along with being an antioxidant, as well as wound-healing effects. Two types of carnosine (dipeptide Zinc and N-acetyl carnosine) have served as an anti-ulcer drug (Cho et al. 1991) and as a cataract agent (Babizhayev et al. 2001), respectively. Their derivatives have been utilized in a variety of ways, including sports nutrition. Initial studies have revealed that carnosine is a forager of:
• Hydroxyl
• Peroxyl
• Superoxide radicals
• Singlet oxygen
Histidine (Carnosine) and Aldehydes
With certain medical conditions, metabolic imbalances and oxidative stresses occur. Heightened levels of oxidized lipids and carbohydrates produce aldehydes in such conditions as myocardial and cerebral ischemia; diabetes; atherosclerosis; neurodegenerative disease processes; and trauma.
Within the majority of tissues, aldehydes are detoxified by catalyzing the oxidation or the decrease of aldehydes, or enzymatic and non-enzymatic conjugation with dipeptides such as glutathione and histidine.
Histidine dipeptides are found in vertebrate tissues. Here they are involved in several physiological functions such as:
• pH buffering
• Metal chelation
• Oxidant and aldehyde scavenging
Histidine peptides (e.g., carnosine) produce adducts with unsaturated aldehydes, and then they react with carbohydrate-derived aldehydes. In-vitro research has revealed that carnosine reduces ischemic injuries; improves glucose control; mitigates any complications in animal diabetics and also in obesity; assists in wound healing; and decreases atherosclerosis.
Initial carnosine treatment has resulted in some impressive findings, including:
• Anti-oxidant properties
• Glycolysis promotion
• Detoxification of reactive aldehydes
• Enhancement of histamine levels
Diseases that are associated with high carbonyl levels may be successfully treated in the future with carnosine and its related histidine peptides.
More on Dipeptide Types
Cyclic dipeptides (CDPs) consist of molecules that are first bacteria, and then used in quorum sensing for humans. Their implications have been found to possibly help control neurodegenerative diseases. In quorum sensing, CDPs relay information about size and population that switches from host symbiosis to virulence. In mammals, CDPs have been found to take action on glial cells for controlling the switch between homoeostatic and inflammatory modes. It is because of their ability to control inflammation through glial cells, thus inducing protective responses within neuronal cells, that research has indicated a potential therapeutic value for a wide variety of inflammatory diseases.
Cyclic dipeptides are some of the simplest peptide derivatives often found in nature. Most cyclic dipeptides seem to have emerged as results of fermentation and food processing. However, many grow from within (endogenous) in members of animal and plants. And while there are five cyclic dipeptides, only one of them, notably cyclo (His-Pro), has been demonstrated to be endogenous to mammals.
Yet cyclo (Leu-Gly), cyclo (Tyr-Arg), and cyclo (Asp-Pro) are structurally related to endogenous peptides Pro-Leu-Gly-NH2 (melanocyte-stimulating hormone release inhibiting factor), Tyr-Arg (kyotorphin), and Val-Pro-Asp-Pro-Arg (enterostatin), respectively, which may serve as precursor peptides. Just as important to note, however, is that further research needs to be conducted to uncover whether these peptides can in fact be caused from processing their corresponding precursors. Most research scientists are confident that bioactive peptides have ample promise for the future to treat diseases and disease processes in humans.
Biological aldehydes are a part of normal metabolism. They are produced during intermediary metabolism as well as lipid and nucleotide synthesis. They are also contained in various foods, even produced in very high levels during unregulated lipid and carbohydrate oxidation. Several disease etiologies are thought to be caused by excessive production and accumulation of aldehydes (oxidative stress). Aldehydes readily react with nucleophilic functional groups that are contained within proteins, lipids and DNA. This is due to the presence of the electrophilic carbonyl group.
Cellular oxidative stress associates with aldehydes, and they are derived from two classes of biological reactions, which are:
1. Polyunsaturated fatty acid oxidation
2. Enzymatic or non-enzymatic metabolism of carbohydrates
Last but certainly not least, Diterpenic resin and dehydroabietic acid (DHA) along with its derivatives have demonstrated enhancement of the inhibition activity of anti-cancer drugs in a variety of cancer cells, including breast, cervical, and hepatocellular cancers. Among the several positive aspects of dipeptides and how they affect disease processes, inhibiting cancer is one of the more promising treatments on the horizon. It's easy to see why more work should be done on dipeptides, as well as why there is so much interest and enthusiasm by those who have uncovered such promising, initial findings regarding dipeptides.
References
1. P. Fürst. Glutamine containing dipeptides: an overview. Clinical Nutrition, 12 (1), 1993, 62-65. [Article].
2. Bellezza I, Peirce MJ, Minelli A. Cyclic dipeptides: from bugs to brain. Trends Mol Med. 2014 Oct;20(10):551-8. [PubMed].
3. Huang XC et al. Synthesis and antitumor activities of novel dipeptide derivatives derived from dehydroabietic acid. Bioorg Med Chem Lett. 2014 Mar 15;24(6):1511-8. [PubMed].
4. Prasad C. Bioactive cyclic dipeptides. Peptides. 1995;16(1):151-64. [PubMed].
5. Santos S et al. Biomedical applications of dipeptides and tripeptides. Biopolymers. 2012;98(4):288-93. [PubMed].
6. Yagasaki M, Hashimoto S. Synthesis and application of dipeptides; current status and perspectives. Appl Microbiol Biotechnol (2008) 81:13–22. [PubMed].