Peptide Bond
All twenty amino acids are characterized by a central alpha carbon covalently bonded to a carboxyl group, an amine group, a hydrogen atom, and a variable R' group. While peptide bonds do not necessarily have to be between two amino acids, most of the time that you see them mentioned, it will be in this context.
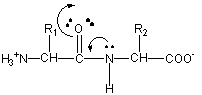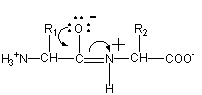
Peptide bond formation mechanism
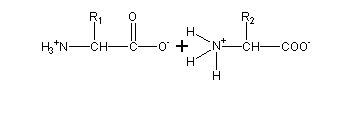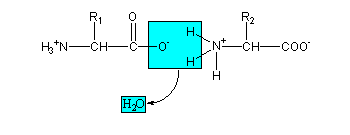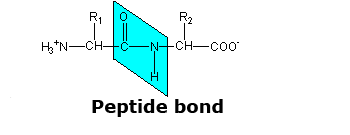
A peptide bond forms when the carboxylic acid group (R-C[O]OH) of one amino acid reacts with the amine group (R-NH2) of another. The resulting molecule is an amide with a C–N bond (R-C(O)-NH-R). This condensation reaction results in a dipeptide, and the release of a water molecule - with a hydroxyl (OH) leaving the carboxyl group, and the hydrogen atom from the amine - with the carboxyl group releasing a hydroxide, and the amine releasing a hydrogen atom. Normally, when we refer to this process in biology, we call it a «dehydration synthesis», since we are building a higher order structure at the expense of a loss of water.
Peptide bond: planar and rigid
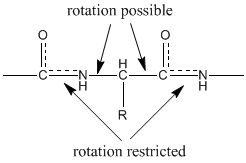
While drawn as a single bond, the peptide bond has partial double bond character that enforces a well-defined flat structure. The carbonyl oxygen has a partial negative (-0.28) charge and is a good hydrogen bond acceptor, while the amide nitrogen is partially positive (+0.28) and a good hydrogen bond donor.
This means that the peptide bond (the C=O and N-H) all reside in a single plane. Because the bond between the carbonyl carbon and the nitrogen has a partial double bond character, rotation around this bond is restricted (88 kJ/mol energy required to rotate). Therefore, the peptide unit is a planar, rigid structure and rotation in the peptide backbone is restricted to the bonds involving the a carbon:
There are two possible conformations of the planar peptide bond: in the trans configuration, the Cα atoms are on opposite sides of the peptide bond and in the cis configuration, the Cα atoms are on the same side of the peptide bond.

In naturally occurring peptides most peptide bonds are in the trans configuration. However, cis forms can occur in peptide bonds that precede a proline residue. In such cases, the cis form is more stable than usual since the proline side-chain offers less of a hindrance. For most peptides the cis-form is about 1000 times less stable than the trans-form.
Breaking a Peptide Bond
One interesting thing to note is that the equilibrium of this reaction lies on the side of hydrolysis rather than synthesis. Hence, the biosynthesis of a peptide bond requires an input of free energy. It may therefore be counterintuitive to learn that peptide bonds are quite stable kinetically: the lifetime of a peptide bond in aqueous solution is approximately 1000 years. Reversing a peptide bond without an enzyme is extremely difficult, thus this process is usually mediated by an enzyme called a protease, such as subtilisin, which is frequently added to laundry detergent to cleave many protein contaminants.
The ability to predictably split peptide bonds is vital to a number of different fields of study. For instance, there is currently much interest in antibody-drug conjugates; these pair fragmented antibodies with pharmacologically active compounds in order to specifically target cancer tumors, among other things.
Absorbance of a peptide bond
The absorbance of peptide bonds, primarily occurring in the UV range around 190-230 nm, is crucial for studying proteins and peptides using UV spectroscopy. This absorbance is linked to the electronic transitions within the amide chromophores of the peptide bond, particularly the n→π* and π→π* transitions. Sensitivity to the local environment enables UV spectroscopy to detect conformational changes in proteins, making it a valuable tool for studying folding dynamics and denaturation. The "280 nm absorbance" is commonly used to estimate peptide and protein concentrations, relying on the contributions of aromatic amino acid residues. In essence, the absorbance of peptide bonds provides insights into the structure, conformation, and concentration of these biomolecules.
Peptide Bonds in Biology
Peptide bonds are made within ribosomes during a process called «translation» to form polypeptides, which then undergo various molecular processing and modification, before folding into a three-dimensional shape, which we call a protein. Proteins can be as small as forty-four amino acids, or as large as thirty-five thousand. A mistake in the translation process can lead to protein mis-folding, and in turn, disease. It is extremely important to understand the physical forces behind a peptide bond, as this allows scientists to design accurate, predictive models of three-dimensional protein structures.